In head-to-head trials
Beovu® exhibited an overall well-tolerated safety profile1
- >1000 patients treated with Beovu® in 2 Phase III trials2
Post-marketing: Retinal vasculitis and/or retinal vascular occlusion have been reported, typically in the presence of intraocular inflammation. The frequency of these events is not known.2
• Uncommon adverse reactions (<1%) included endophthalmitis, blindness, retinal artery occlusion, retinal detachment, conjunctival hyperemia, lacrimation increased, abnormal sensation in eye, detachment of retinal pigment epithelium, vitritis, anterior chamber inflammation, iridocyclitis, anterior chamber flare, corneal edema, and vitreous hemorrhage.2
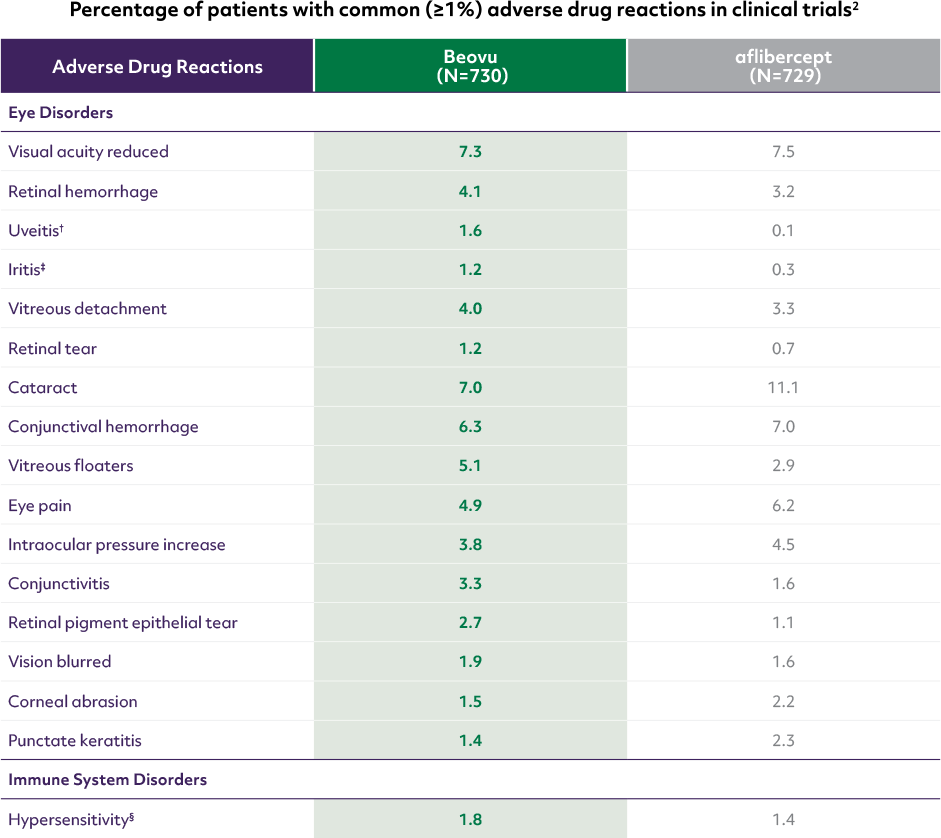
*Beovu® arms in HAWK: 3 mg (n=358), 6 mg (n=360); in HARRIER: 6 mg (n=370).1,3
†In a clinical trial (HAWK), the incidence was 2/60 (3.3%) in Japanese patients on Beovu and 6/300 (2.0%) in all other patients.2
‡In a clinical trial (HAWK), the incidence was 4/60 (6.7%) in Japanese patients on Beovu and 5/300 (1.7%) in all other patients.2
§Including urticaria, rash, pruritus, and erythema.2
REFERENCES:
-
Data on file. RTH258-C002 Clinical Study Report. Novartis; 2018.
-
Data on file. RTH258 Core Data Sheet. Novartis; 2020.
-
Data on file. RTH258-C001 Clinical Study Report. Novartis; 2018.
Beovu® SmPC
Rate this content:
11/20 GLOPH/BRO/0307
