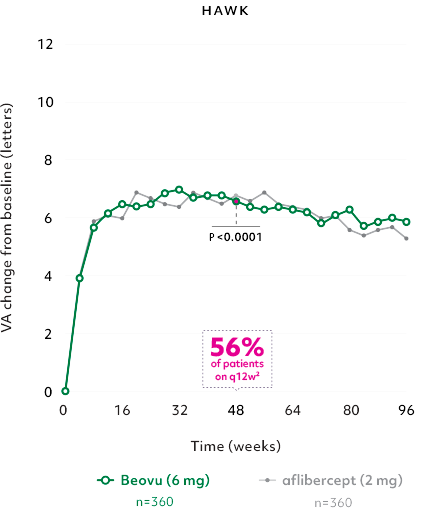
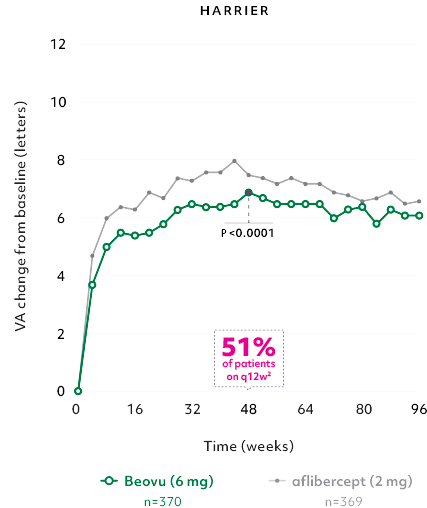
Robust vision gains1
- Gains achieved with a majority of patients on q12w2
- The vision gains was maintained at Week 962
Mean change in VA of Beovu® vs aflibercept2,3
q8w=treatment every 8 weeks.
The primary efficacy endpoint for the studies was the change from baseline in Best Corrected Visual Acuity (BCVA) to Week 48 as measured by the Early Treatment Diabetic Retinopathy Study (ETDRS) Letter Score with the primary objective to demonstrate noninferiority of Beovu vs aflibercept.2
Beovu (q12w/q8w) demonstrated noninferiority in VA to aflibercept 2 mg (fixed q8w).2
*No hypothesis testing was performed after Week 48.
REFERENCES
1. Dugel PU, Koh A, Ogura Y, et al; on behalf of the HAWK and HARRIER Study Investigators. HAWK and HARRIER: Phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72-123.
2 .Summary of Product Characteristics. Novartis; 2020.
3. Data on file. BCVA Data. Novartis; 2019.
Rate this content: