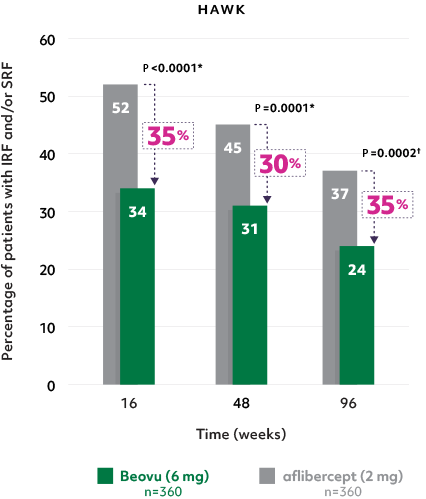
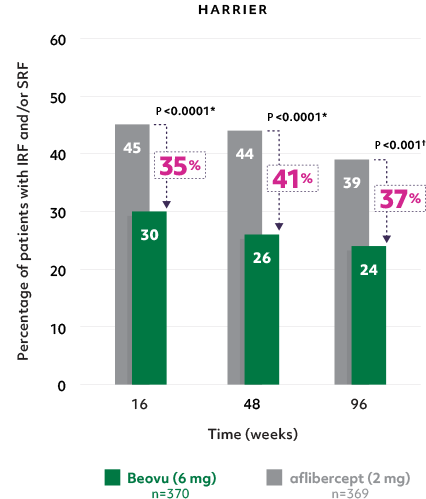
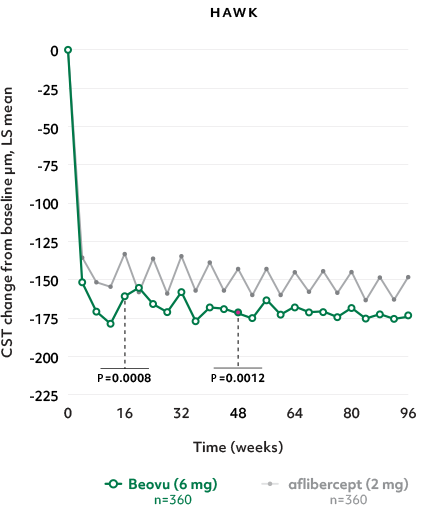
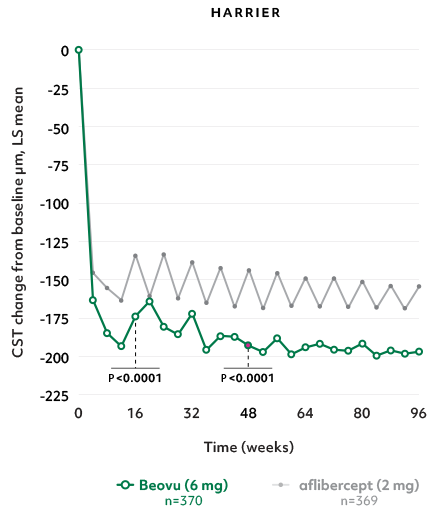
Superior fluid resolution that lasts1
• Beovu® outperformed aflibercept, with significantly fewer patients with IRF and/or SRF at Weeks 16 and 481
Patients with IRF and/or SRF on Beovu vs aflibercept1-4
Early superiority that lasts1
- Beovu outperformed aflibercept with superior CST reductions at Weeks 16 and 481*
- The difference was maintained at Week 96 (P=0.0115 in HAWK, P<0.0001 in HARRIER)4,5†‡
CST Reductions for Beovu vs aflibercept1-4
LS=least squares; OCT=optical coherence topography.
*Secondary endpoint in HAWK and HARRIER, confirmatory analysis in HAWK only (1-sided P values for superiority of Beovu).2
†Secondary endpoints in HAWK and HARRIER; 2-sided P values.1 ‡No hypothesis testing was performed after Week 48.
Reduce thickness. Gain letters1
- Most patients experienced vision gains and reductions in retinal thickness at Week 481
Averate patient CST response*3
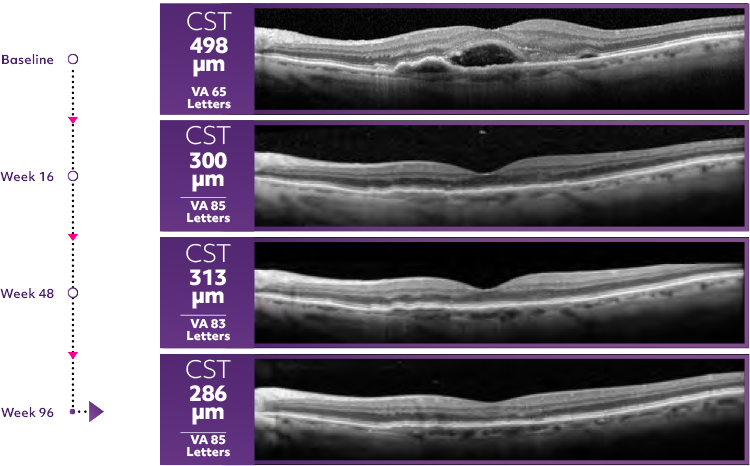
OCT scan of Beovu patient on q12w
*OCT scans are a representation of mean patient response in HAWK.
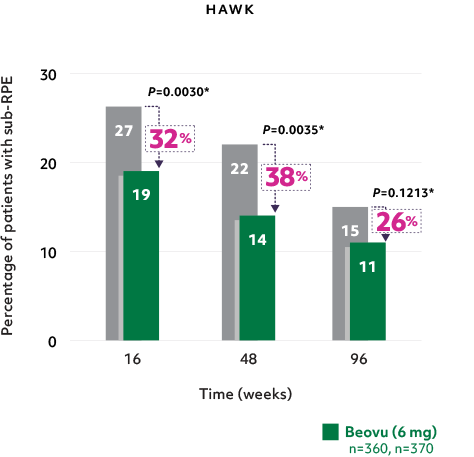
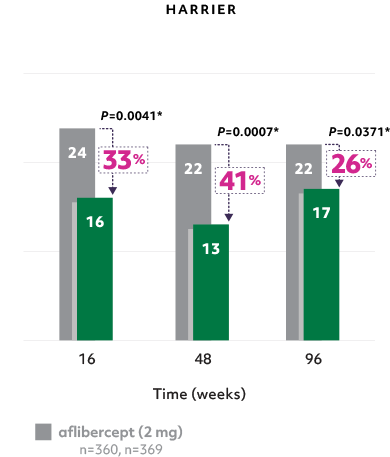
Fewer patients with sub-RPE fluid5
- Reductions in sub-RPE fluid were demonstrated at Weeks 16 and 485*
- The difference was maintained at Week 965*
Fewer patients with sub-RPE on Beovu vs aflibercept1-4
*Secondary endpoints in HAWK and HARRIER; 2-sided P values.2,3
REFERENCES
1. Dugel PU, Koh A, Ogura Y, et al; on behalf of the HAWK and HARRIER Study Investigators. HAWK and HARRIER: Phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72-123.
2. Data on file. RTH258-C001 Clinical Study Report. Novartis; 2018.
3. Data on file. RTH258-C002 Clinical Study Report. Novartis; 2018.
4. Data on file. RTH258-C001 & C002 IRF/SRF to 96. Novartis; 2018.
5. Summary of Product Characteristics. Novartis; 2020.
6. Data on file. RTH258 CST Data. Novartis; 2018.
Rate this content: