HAWK and HARRIER: Phase III Pivotal Trials1
HAWK & HARRIER: 2 head-to-head, 96-week clinical trials
for Beovu® vs aflibercept1
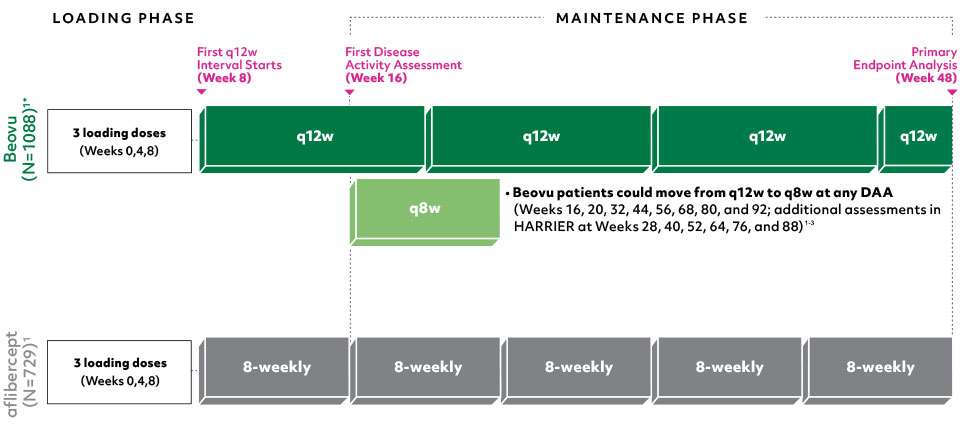
*Beovu® arms in HAWK: 3 mg (n=358), 6 mg (n=360); in HARRIER: 6 mg (n=370).1
Maintenance phase continued through study end (Week 96)1
- No hypothesis testing was performed after Week 481
Primary endpoint: Change from baseline in VA at Week 48, measured by ETDRS letter score1
Disease Activity Assessments (DAAs): Anatomical and functional parameters† used to determine if patients remained on q12w intervals1,4
† Comprehensive DAA criteria at Week 16: Decrease in VA of ≥5 letters compared with baseline; decrease in VA of ≥3 letters and CST increase ≥75 μm compared with Week 12; decrease in VA of ≥5 letters due to wet AMD disease activity compared with Week 12; new or worse IRF/IRC compared with Week 12. DAA criteria at Weeks 20, 32, and 44: Decrease in VA of ≥5 letters compared with Week 12.
Select baseline characteristics
- Simple mean age: 76 years1
- Simple mean VA: 61 letters read; comparable between treatment arms2-4
- Baseline ocular characteristics for study eye were well balanced across treatment arms2,3*
- 73% of patients with unilateral wet AMD3,4
- 92% of patients were diagnosed ≤ 3 months prior to study entry2,3
AMD=age-related macular degeneration; CNV=choroidal neovascularization, DAAs=disease activity assessments; ETDRS=Early Treatment Diabetic Retinopathy Study; IRC=intraretinal cysts; IRF=intraretinal fluid; sub-RPE=sub-retinal epithelium; SRF=sub-retinal fluidVA=visual acuity (VA is used to describe best corrected visual acuity [BCVA] as identified in study protocol).
*Baseline characteristics include time since diagnosis, VA (letters read), % of unilateral vs bilateral wet AMD, CST-total, presence of fluid (IRF, SRF, sub-RPE), type of CNV, and area associated with CNV lesion.3
The HAWK and HARRIER trials video
REFERENCES
-
Summary of Product Characteristics. Novartis; 2020.
-
Data on file. RTH258-C001. Novartis; 2019.
-
Data on file. RTH258-C002. Novartis; 2019.
-
Dugel PU, Koh A, Ogura Y, et al; on behalf of the HAWK and HARRIER Study Investigators. HAWK and HARRIER: Phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72-123.
Beovu® SmPC
Rate this content:
11/20 GLOPH/BRO/0307
