Longer intervals, less treatment burden1-4
The only anti-VEGF recommended to start eligible patients on q12w intervals immediately after loading
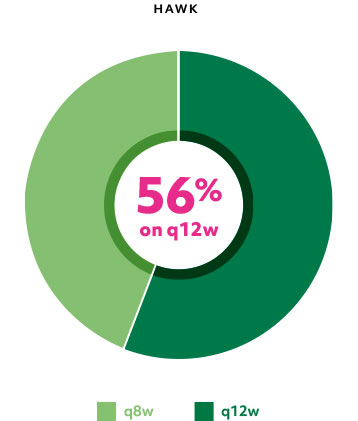
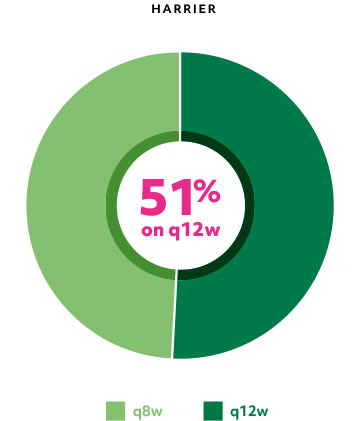
Majority of patients were on q12w with Beovu at Week 481*
-
Patients on q12w at Week 48 had an 82% (HAWK) and 75% (HARRIER) probability of remaining on q12w through Week 961
q8w=treatment every 8 weeks; q12w=treatment every 12 weeks
*All remaining patients were maintained on q8w.1
REFERENCES:
-
Summary of Product Characteristics. Novartis; 2020.
-
Data on file. RTH258-C002 Clinical Study Report. Novartis; 2018.
-
Boyle J, Vukicevic M, Koklanis K, Itsiopoulos C, Rees G. Experiences of patients undergoing repeated intravitreal anti-vascular endothelial growth factor injections for neovascular age-related macular degeneration. Psychol Health Med. 2018;23(2):127-140.
-
Prenner JL, Halperin LS, Rycroft C, Hogue S, Williams Liu Z, Seibert R. Disease burden in the treatment of age-related macular degeneration: findings from a time-and-motion study. Am J Ophthalmol. 2015;160(4):725-731.e1. doi:10.1016/j.ajo.2015.06.023.
-
Lucentis Summary of Product Characteristics. Dublin, Ireland: Novartis; 2019.
-
Eylea Summary of Product Characteristics. Leverkusen, Germany: Bayer AG; 2019.
Beovu® SmPC
Rate this content:
11/20 GLOPH/BRO/0307