Onboarding
Slow down SPMS with Active Disease. It’s time for Mayzent1
For guidance on a successful switch to MAYZENT, select the MS treatment your patients are currently receiving from the tabs below1

No dosing gap is required if the patient's lymphocyte counts are normal.2

No dosing gap is required if the patient's lymphocyte counts are normal2,3

Patients should undergo an accelerated elimination of 11-14 days or a >3.5-month safety dosing gap.2,4,5

Patients should observe a safety dosing gap of 8-12 weeks before initiating MAYZENT.6

Switching patients to MAYZENT from alemtuzumab is not recommended2

Patients should undergo at least a 14-week washout period.2

Depending on lymphocyte count recovery, a safety gap of 6-8 months should be considered.7*

Patients can initiate MAYZENT with no dosing gap.8†
AV=atrioventricular; MS=multiple sclerosis; S1P=sphingosine 1-phosphate; SPMS=secondary progressive MS.
*Based on median time to lymphocyte recovery of 28 weeks, as reported in the cladribine label.7
†MAYZENT and fingolimod reduce lymphocyte count to 20%-30% of baseline through S1P receptor modulation; no major additional reduction is expected after a direct switch from fingolimod to MAYZENT. The transient effects of fingolimod and MAYZENT on heart rate during initial dosing derive from initial S1P receptor agonism followed by S1P receptor internalization and desensitization to heart rate effects. Direct switch from fingolimod to MAYZENT should not require a titration dose and starting with the maintenance dose (2 mg or 1 mg) is not expected to result in heart rate or AV conduction effects.5,8,9
Find my MAYZENT representative
Search by entering your main practice's address in our map tool
Show more
Reference: 1. MAYZENT [Summary of Product Characteristics]. Novartis Pharmaceuticals AG. 2021. 2. Data on file. CBAF312AUS02 protocol amendment. Novartis Pharmaceuticals Corp. [January 15, 2020.] 3. Tecfidera [package insert]. Cambridge, MA: Biogen Inc; January 2021. 4. Aubagio [package insert]. Cambridge, MA: Genzyme Corporation; April 2021. 5. Mayzent [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corp; January 2021. 6. Sellner J, Rommer PS. A review of the evidence for a natalizumab exit strategy for patients with multiple sclerosis. Autoimmun Rev. 2019;18(3):255-261. 7. Mavenclad [package insert]. Darmstadt, Germany: Merck KGaA; April 2019. 8. Data on file. Switching safely from Gilenya to siponimod. Novartis Pharmaceuticals Corp. [June 27, 2019.] 9. Gilenya [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corp; December 2019.
Beginning patients on once-daily MAYZENT follows a well-defined initiation protocol
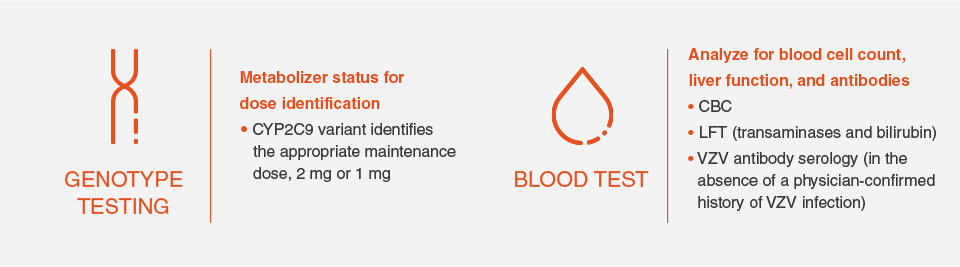
Additional tests for patients

AV=atrioventricular; CBC=complete blood count; ECG=electrocardiogram; LFT=liver function test; VZV=varicella zoster virus.
Find my MAYZENT representative
Search by entering your main practice's address in our map tool
Show more
Reference: 1. MAYZENT [Summary of Product Characteristics]. Novartis Pharmaceuticals AG. 2021.
A tailored titration schedule helps patients safely reach the maintenance dose
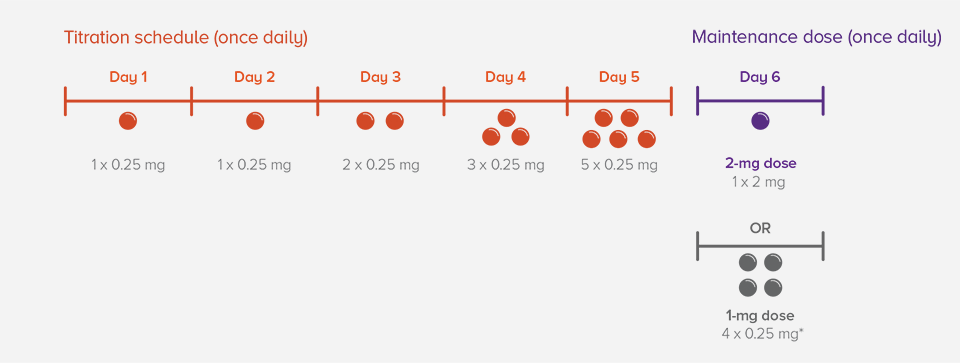
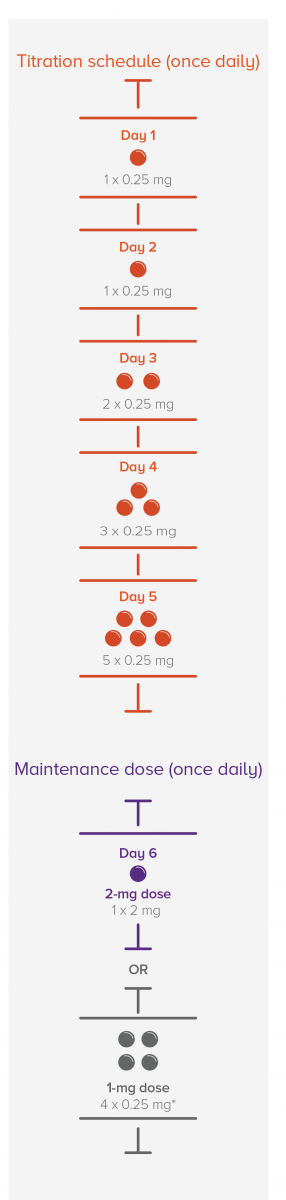
If a dose is missed during treatment initiation
|
*Recommended maintenance dose for patients with CYP2C9*2*3 or *1*3 genotype.
Find my MAYZENT representative
Search by entering your main practice's address in our map tool
Show more
Reference: 1. MAYZENT [Summary of Product Characteristics]. Novartis Pharmaceuticals AG. 2021.
Patients should be monitored while taking MAYZENT
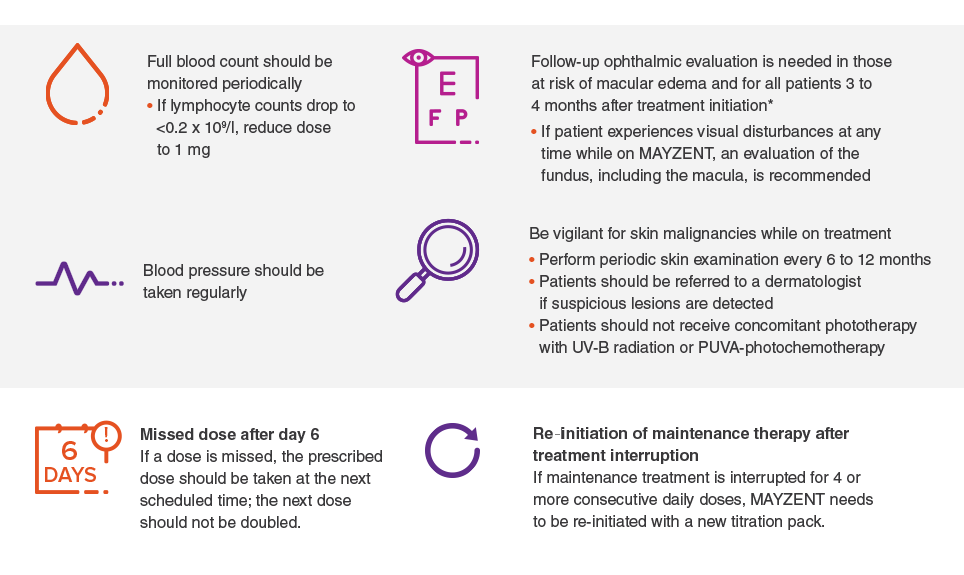
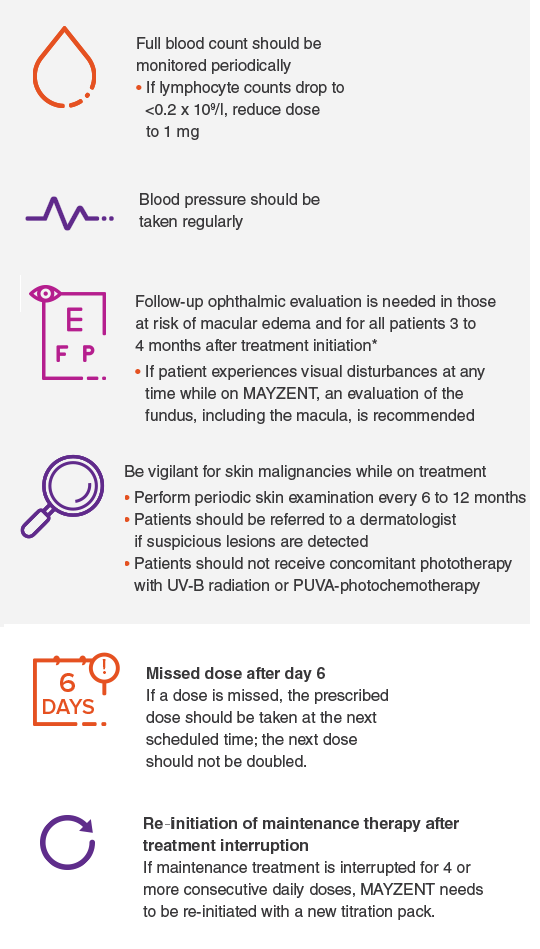
*It is recommended that MAYZENT be discontinued if a patient develops macular edema.
Find my MAYZENT representative
Search by entering your main practice's address in our map tool
Show more
References: 1. MAYZENT [Summary of Product Characteristics]. Novartis Pharmaceuticals AG. 2021.
Rate this content:
GLNS/SIPO/117317

 Reporting suspected adverse reactions after authorization of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions at [Placeholder for web address of local country's national reporting system for adverse reactions].
Reporting suspected adverse reactions after authorization of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions at [Placeholder for web address of local country's national reporting system for adverse reactions].